“What’s up with That?”
By Patrick C. Cox, Jr

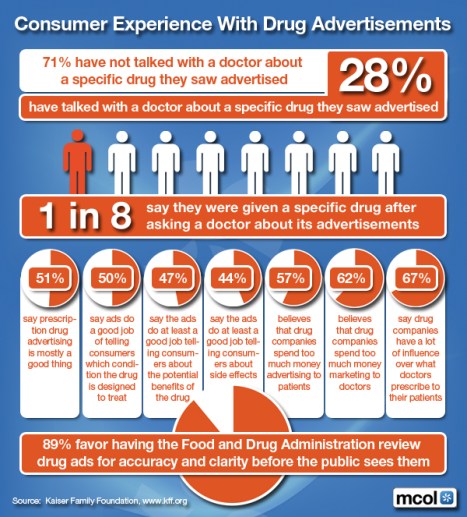
How many times have you viewed the Lipitor® “educational” commercial featuring Dr. Robert Jarvik?
If you are like me, probably often since Pfizer appears set on getting their money’s worth from the production. The spot pops up everywhere it seems. Pretty impressive with the rowing, the lake, and natural beauty along with a heartfelt pitch from Jarvik on what a difference Lipitor® has made in his life.
He shares his thoughts that Lipitor® is one of the most researched drugs and he’s glad that he takes it as a doctor, and a dad.
Well, the commercial may have run one time too many. Questions have been raised by consumers and now congressional figures as to Jarvik’s credibility and his Lipitor® endorsement.
For example, he never pursued a medical internship, is not licensed to practice and can’t legally prescribe.
Of course, Jarvik has been recently defending his status as a scientist and his role in simply educating the public in the ad. But, his revelation as a past Lipitor® patient, with implied personal endorsement, could certainly make patients feel they’re missing something if they too aren’t taking the compound.

Assessment
Of course, we all know the real reason behind the ad. Pfizer has invested millions hoping Jarvis’s personal endorsement will get patients saying to themselves, “Hey, Dr. Jarvik prescribes it for his patients and takes it himself, how come I’m not on it?”
The next step they’re hoping for is for them to ask their “prescribing” physician the same thing.
Assessment
Well that’s the problem, isn’t it? Lipitor® is a good drug, but it’s not for everyone. And, only doctors should know what’s best for their own patient’s; right?
So, should drug companies be held accountable for these ads and/or provide more disclosure to the public?
In other word’s, should Pfizer have told us that Jarvik isn’t really a doctor, can’t and isn’t prescribing anything to anyone – and wait a second – did he really even take Lipitor® at all?
Guess only his “prescribing” physician would know, for sure!
Conclusion
Your thoughts and comments on this ME-P are appreciated. Feel free to review our top-left column, and top-right sidebar materials, links, URLs and related websites, too. Then, subscribe to the ME-P. It is fast, free and secure.
Speaker: If you need a moderator or speaker for an upcoming event, Dr. David E. Marcinko; MBA – Publisher-in-Chief of the Medical Executive-Post – is available for seminar or speaking engagements. Contact: MarcinkoAdvisors@msn.com
OUR OTHER PRINT BOOKS AND RELATED INFORMATION SOURCES:



Filed under: Drugs and Pharma | Tagged: Big Pharma and Drugs | 5 Comments »